Adverse events following quadrivalent meningococcal CRM-conjugate vaccine Menveo reported to the Vaccine Adverse Event Reporting system VAERS 2010-2015. Published online by Cambridge University Press.
 Meningococcal Vaccination Recommendations And Timing Of Administration Download Scientific Diagram
Meningococcal Vaccination Recommendations And Timing Of Administration Download Scientific Diagram
In the past 10-15 years.
Quadrivalent meningococcal vaccine. A Phase III randomized study. PierreLandryhospvdch Meningoccocal meningitis is still a severe disease. Meningococcal quadrivalent vaccines protect against 4 types of meningococcal bacteria.
Why should I get the Men-C-ACYW-135 vaccine. A booster dose is recommended about 5 years after the first dose around age 16. What are meningococcal quadrivalent vaccines.
Meningitidis strains each conjugated to diphtheria toxoid. 2017 Mar 2735 141758-1763. Siberry GK 1 Warshaw MG Williams PL Spector SA Decker MD Jean-Philippe P Yogev R Heckman BE Manzella A Roa J Nachman S Lujan-Zilbermann J.
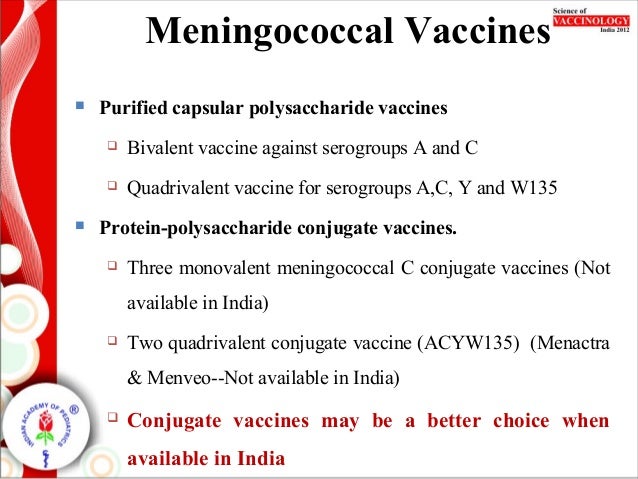
Meningococcal conjugate or MenACWY vaccines Menactra and Menveo Serogroup B meningococcal or MenB vaccines Bexsero and Trumenba. Because they provide longer lasting protection against disease. There are 2 types of meningococcal vaccines available in the United States.
To evaluate the real-world safety evidence of meningococcal conjugate vaccines. Safety and immunogenicity of quadrivalent meningococcal conjugate vaccine in 2- to 10-year-old human immunodeficiency virus-infected children. Two quadrivalent meningococcal conjugate vaccines MenACWY that prevent invasive meningococcal disease caused by N.
IMPAACT P1065 Protocol Team. Quadrivalent meningococcal vaccine was added to the recommended immunization schedule in 2005. What is the Men-C-ACYW-135 vaccine.
Potential alterations in the ecological niche of meningococcus such that other bacteria that will replace meningococcus as carriage of this organism decreases. The potential occurrence of serogroup replacement. Vaccines can help prevent meningococcal disease which is any type of illness caused by Neisseria meningitidis bacteria.
The first vaccine -- meningococcal polysaccharide vaccine or MPSV4 -- was approved in 1978. Meningitidis serogroups A C Y and W have been licensed in the US. This should be offered at least 6 months after vaccination with polysaccharide meningococcal vaccine.
Meningococcal quadrivalent conjugate vaccine is 4 weeks regardless of which vaccine is given first. Study on a Quadrivalent Meningococcal Conjugate Vaccine MenACYW Conjugate Vaccine Compared to a Meningococcal Reference Vaccine and When Given Alone or With Two Other Vaccines in Healthy Adolescents The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The quadrivalent conjugate meningococcal vaccine is a routine childhood vaccination given to adolescents preferably at age 11 or 12 years with a booster dose at age 16 years see Table.
Immunization coverage of individual quadrivalent meningococcal vaccines and the impact on carriage and herd immunity. This vaccine not only provides protection against serogroup C but also against 3 additional strains that cause meningococcal disease serogroups A Y and W-135. Quadrivalent meningococcal conjugate vaccine MenACWY-D Menactra Sanofi contains 4 µg of each polysaccharide derived from the capsules of group A C W and Y N.
While both types of vaccines are approved by Health Canada the conjugate vaccines are used in BC. Article in French Landry P1. One dose of the quadrivalent vaccine is recommended for children and adolescents around age 11 or 12.
Quadrivalent meningococcal conjugate vaccine. 1Centre de vaccinations et médecine des voyages PMU 1011 Lausanne. The meningococcal conjugate quadrivalent Men-C-ACYW-135 vaccine is approved by Health Canada and provides protection against meningococcal disease that is caused by the strains A C Y and W-135.
Conjugate C vaccine for grade 7 students with a quadrivalent meningococcal conjugate vaccine Men-C-ACYW-135 Menactra. Other components include sodium chloride and sodium phosphate. Its made with the antigens contained in the outer polysaccharide or.
Recommended Immunization Schedule for Ages 718 Years. The vaccine works by causing your body to produce its own protection antibodies against the disease. In Switzerland the predominant serotypes are serotype B and C but this could change as other.
Immunogenicity and Safety of a Quadrivalent Meningococcal Tetanus Toxoid-Conjugate Vaccine MenACYW-TT Administered Concomitantly with Other Paediatric Vaccines in Toddlers. We systematically reviewed published studies conducted in the US. Meningococcal tetanus toxoid conjugate quadrivalent vaccine is an active immunizing agent used to prevent infection caused by certain groups of meningococcal bacteria Neisseria meningitides.
Epub 2017 Mar 3. This vaccine is given by needle. Eligible individuals previously vaccinated with a polysaccharide meningococcal vaccine should be given meningococcal quadrivalent conjugate.
The vaccines are either polysaccharide or conjugate vaccines. Types A C Y and W-135.
 Suggested Recommendations Of Use Of Meningococcal Vaccine In South Africa Download Table
Suggested Recommendations Of Use Of Meningococcal Vaccine In South Africa Download Table
 Prevention Of Meningococcal Infection In The United States Current Recommendations And Future Considerations Sciencedirect
Prevention Of Meningococcal Infection In The United States Current Recommendations And Future Considerations Sciencedirect
 Immunogenicity And Safety Of A Quadrivalent Meningococcal Polysaccharide Crm Conjugate Vaccine In Infants And Toddlers International Journal Of Infectious Diseases
Immunogenicity And Safety Of A Quadrivalent Meningococcal Polysaccharide Crm Conjugate Vaccine In Infants And Toddlers International Journal Of Infectious Diseases
 Meningococcal Polysaccharide A O Acetylation Levels Do Not Impact The Immunogenicity Of The Quadrivalent Meningococcal Tetanus Toxoid Conjugate Vaccine Results From A Randomized Controlled Phase Iii Study Of Healthy Adults Aged 18 To
Meningococcal Polysaccharide A O Acetylation Levels Do Not Impact The Immunogenicity Of The Quadrivalent Meningococcal Tetanus Toxoid Conjugate Vaccine Results From A Randomized Controlled Phase Iii Study Of Healthy Adults Aged 18 To
 Meningococcal Conjugate Vaccines Download Table
Meningococcal Conjugate Vaccines Download Table
 Safety And Immunogenicity Of A Pentavalent Meningococcal Conjugate Vaccine Containing Serogroups A C Y W And X In Healthy Adults A Phase 1 Single Centre Double Blind Randomised Controlled Study The Lancet Infectious
Safety And Immunogenicity Of A Pentavalent Meningococcal Conjugate Vaccine Containing Serogroups A C Y W And X In Healthy Adults A Phase 1 Single Centre Double Blind Randomised Controlled Study The Lancet Infectious
 Subject Disposition Flowchart Menacwy Crm Quadrivalent Meningococcal Download Scientific Diagram
Subject Disposition Flowchart Menacwy Crm Quadrivalent Meningococcal Download Scientific Diagram
 Meningococcal Quadrivalent Vaccines 18 101 145 Download Table
Meningococcal Quadrivalent Vaccines 18 101 145 Download Table
 Meningococcal Conjugate Vaccine Menactra Meningococcal Groups A C Y And W 135 Polysaccharide Diphtheria Toxoid Conjugate Vaccine
Meningococcal Conjugate Vaccine Menactra Meningococcal Groups A C Y And W 135 Polysaccharide Diphtheria Toxoid Conjugate Vaccine
 Nimenrix Quadrivalent Meningococcal Polysaccharide Conjugate Vaccine
Nimenrix Quadrivalent Meningococcal Polysaccharide Conjugate Vaccine
 Meningococcal Vaccine Breakout
Meningococcal Vaccine Breakout
 Effect Of A Quadrivalent Meningococcal Acwy Glycoconjugate Or A Serogroup B Meningococcal Vaccine On Meningococcal Carriage An Observer Blind Phase 3 Randomised Clinical Trial The Lancet
Effect Of A Quadrivalent Meningococcal Acwy Glycoconjugate Or A Serogroup B Meningococcal Vaccine On Meningococcal Carriage An Observer Blind Phase 3 Randomised Clinical Trial The Lancet
![]() Meningococcal Vaccine Diseases And Conditions Pediatric Oncall
Meningococcal Vaccine Diseases And Conditions Pediatric Oncall

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.