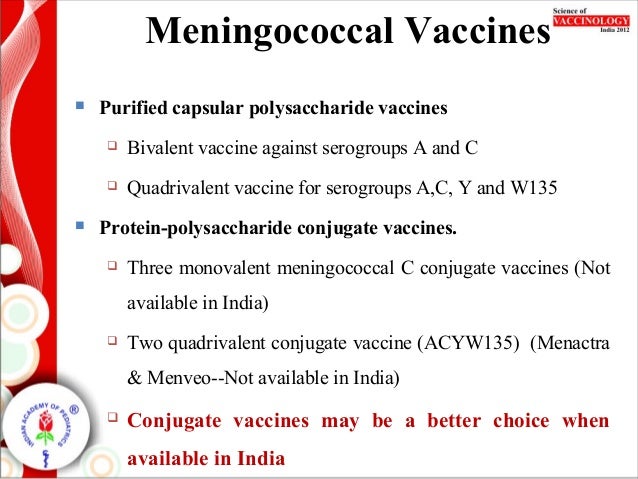
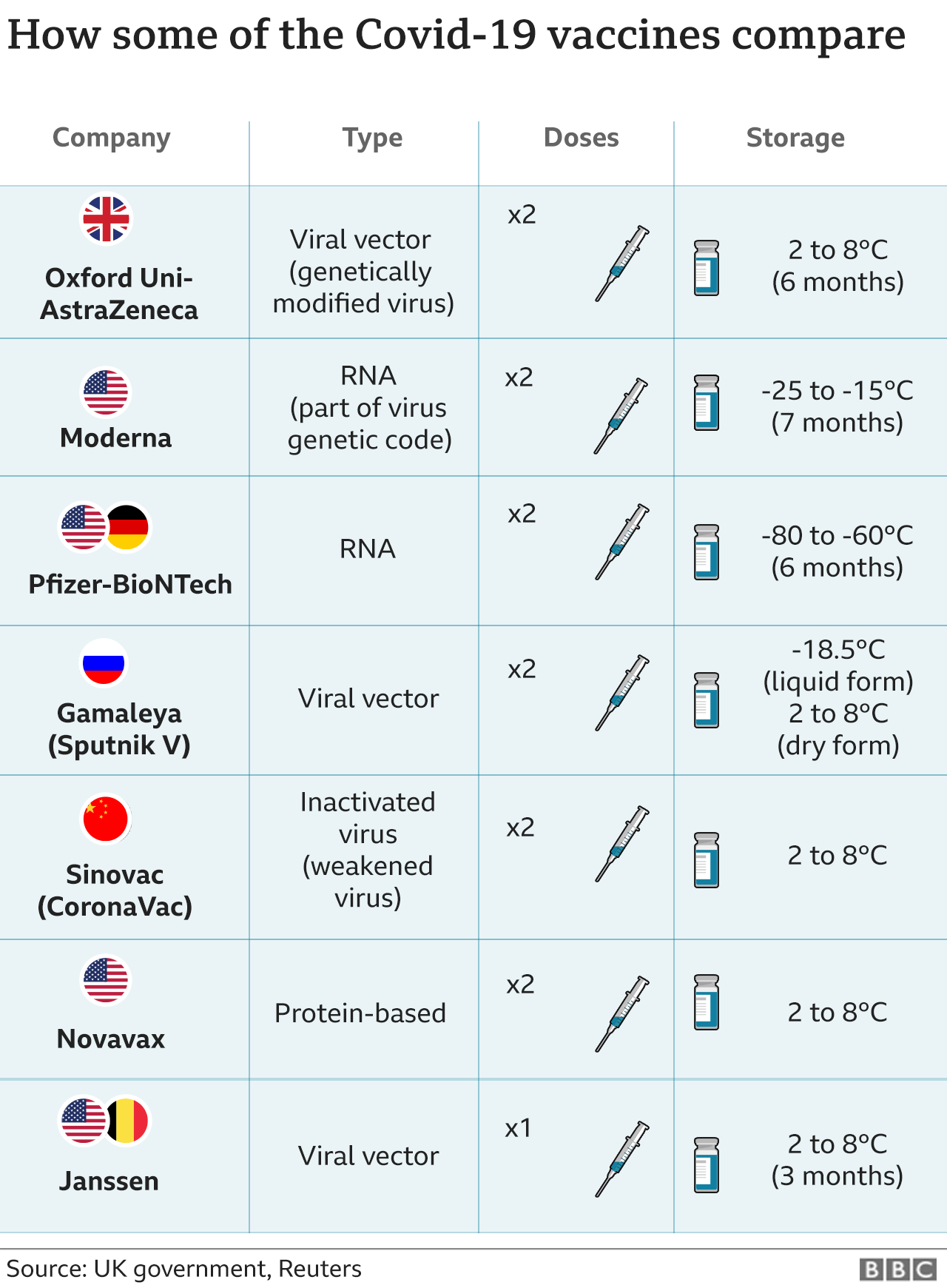
Instead of injecting a weakened form of a virus or bacteria into the body DNA and RNA vaccines use part of the virus own genes to stimulate an immune response. Some vaccines do contain human DNA Vaccines for chickenpox rubella and hepatitis A are created using human embryo cells according to an article from the Childrens Hospital of Philadelphia.
 Proposed Mechanism Of Dna Vaccines Download Scientific Diagram
Proposed Mechanism Of Dna Vaccines Download Scientific Diagram
It works by giving the body instructions to produce a protein.
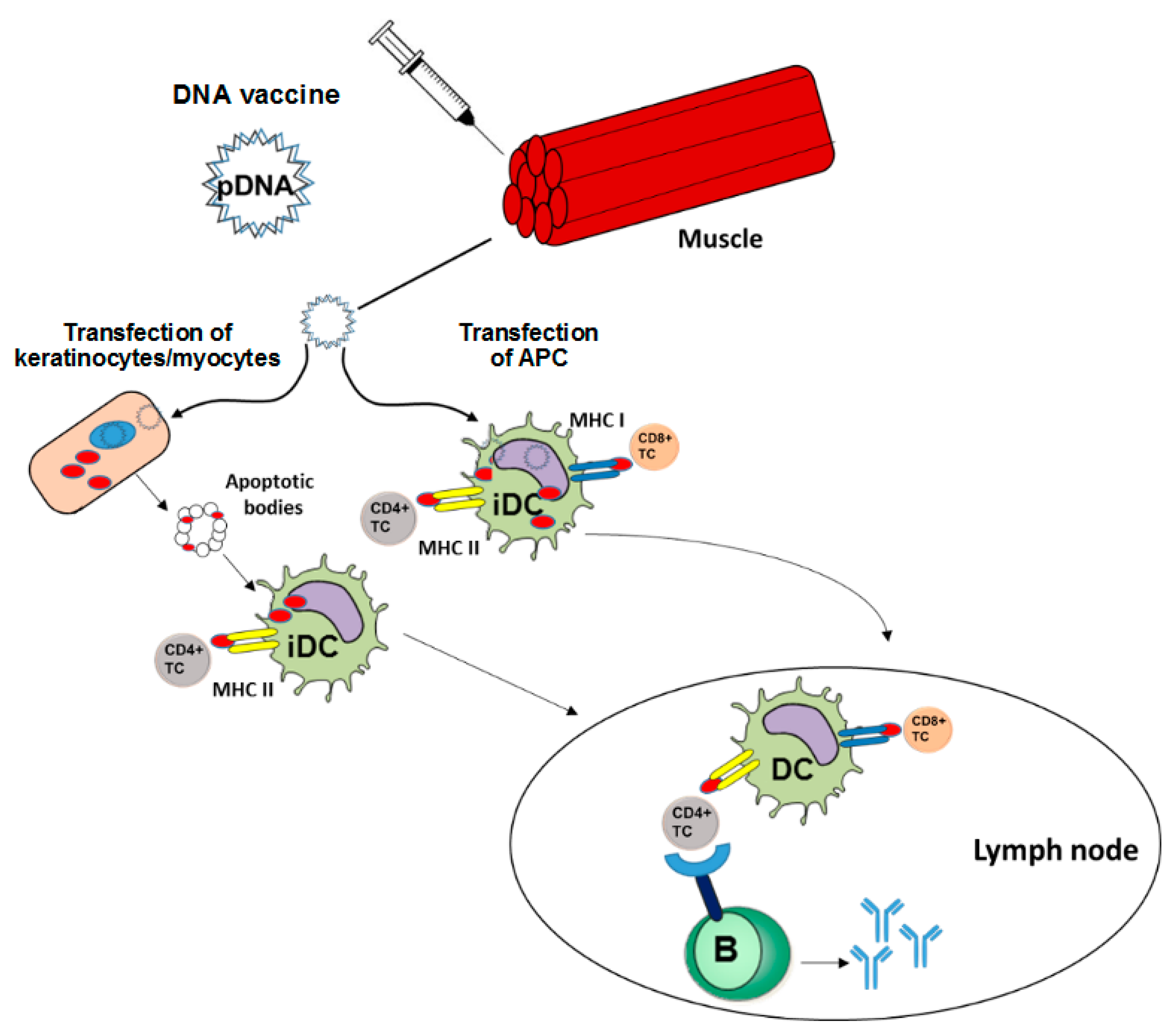
What is dna vaccine. This review provides a comparison of the theoretical issues and experimental findings for plasmid DNA and mRNA vaccine technologies. Transfection is how your immune. DNA vaccines which are often referred to as the third-generation vaccines use engineered DNA to induce an immunologic response in the host against bacteria parasites viruses and potentially.
Instead of using a weakened or dead version of a virus mixed with protein and other ingredients the main agent in a DNA vaccine is made from part of the virus own genetic information. The recent COVID-19 vaccines actually use mRNA from the virus itself in a rather sneaky way. According to Mirus Bio a medical corporation dedicated to the study of transfection it can be defined as the introduction of DNA or RNA into eukaryotic cells.
While both have been under development since the 1990s in recent years significant excitement has turned to mRNA despite the licensure of several veterinary DNA vaccines. A DNA vaccine uses a gene from a virus or bacteria to stimulate the immune system. Proteins are the building blocks that.
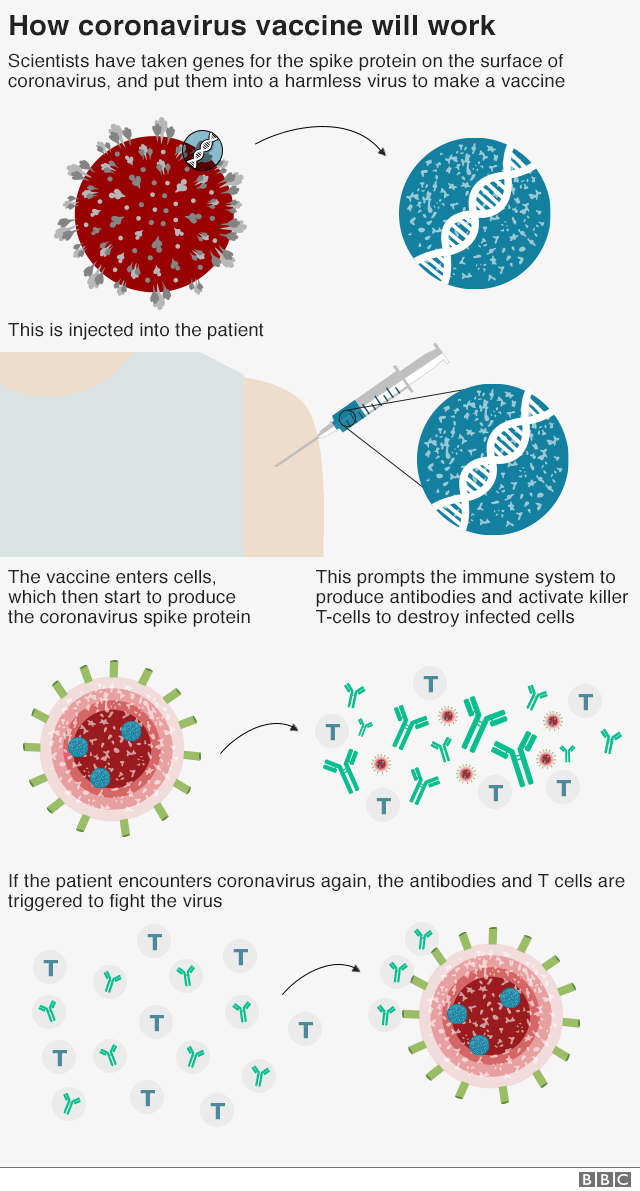
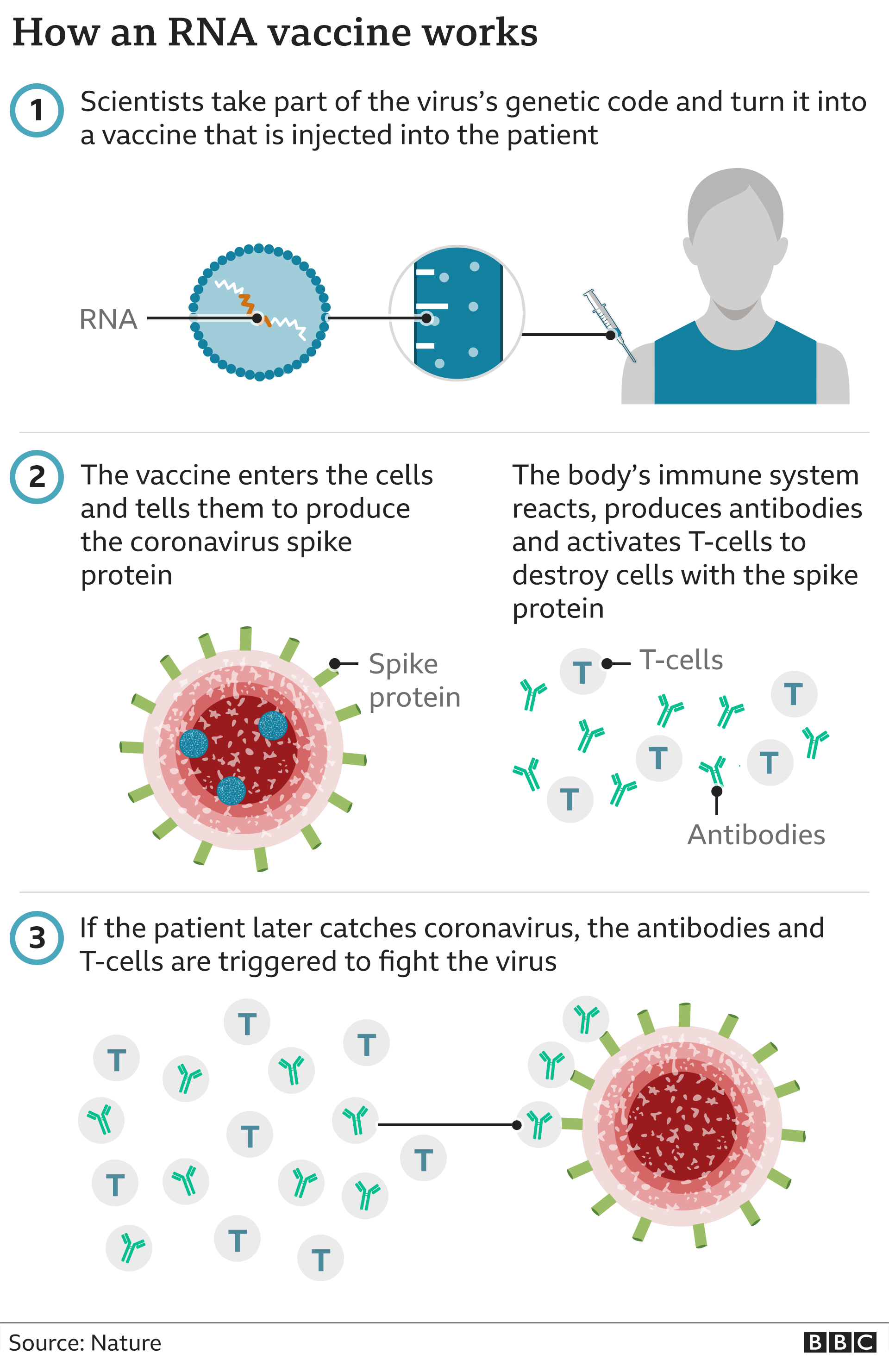
In this type of vaccine a gene from a virus or bacterial is used to stimulate the immune system. Injecting RNA into a person doesnt do anything to the DNA of a human cell says Prof Jeffrey Almond of Oxford University. A DNA or RNA vaccine has the same goal as traditional vaccines but they work slightly differently.
Other organisms including bacteria and viruses also have DNA andor RNA. But there are crucial differences between DNA which carries all of the information we inherited from our parents and mRNA which the Moderna and PfizerBioNTech vaccines are made of. When the DNA vaccine is administered to a patient the machinery in their cells makes a viral or bacterial protein which their immune system recognises as being foreign to.
When the DNA vaccine is administered to a patient their cells machinery produces a viral or. DNA plasmid vector vaccines carry the genetic information encoding an antigen allowing the. DNA vaccines favor a cell-mediated immune response.
Usually a vaccine uses a weakened or damaged version of a virus so that your body can have a practice run of fighting it. DNA vaccines What is a DNA vaccine. RNA is like a temporary photocopy of DNA and is used to make proteins.
They use a modified benign virus to ferry in DNA. The mRNA vaccines like those developed by Pfizer-BioNTech and Moderna dont use DNA. DNA can be a concern related to vaccines in two ways because it is the vacciness active ingredient such as in adenovirus-based vaccines or as a manufacturing byproduct following growth of vaccine virus in human fetal cells.
The other two vaccines that have made it to this stage of clinical testing come from AstraZeneca and Janssen. Vaccination consists of stimulating the immune system with an infectious agent or components of an infectious agent modified in such a manner that no harm or disease is caused but ensuring that when the host is confronted with that infectious agent the immune system can adequately neutralize it before it causes any ill effect.