Due to similarities in the clinical presentation of CAR-T cytokine release syndrome and the hyperinflammatory state of severe COVID-19 interest in tocilizumab for severe COVID-19 developed early in the pandemic Mehta March 2020. Tocilizumab and sarilumab are monoclonal antibodies that inhibit both membrane-bound and soluble interleukin-6 receptors and are used to treat inflammatory conditions such as rheumatoid arthritis.
 Tocilizumab Calms The Inflammatory Storm Through Blocking Il 6 Receptors Download Scientific Diagram
Tocilizumab Calms The Inflammatory Storm Through Blocking Il 6 Receptors Download Scientific Diagram
Up until recently significant uncertainty remained regarding clinical use.
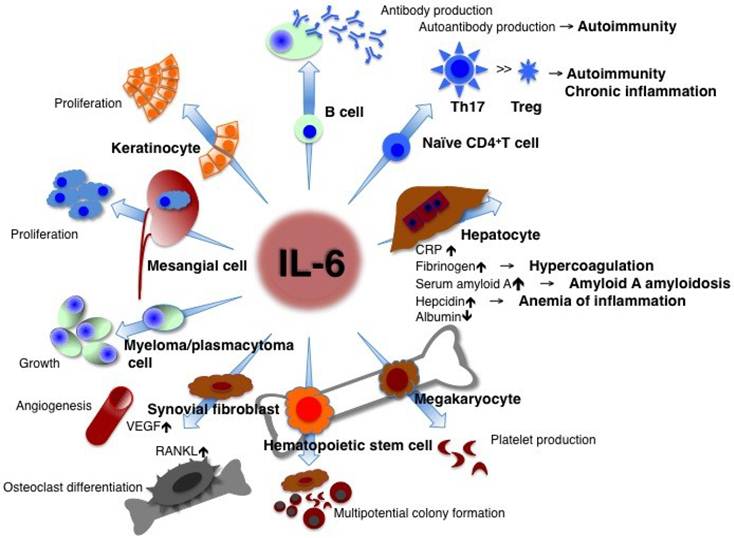
Tocilizumab il 6. Interception of the IL-6 signaling pathway could thus represent a new treatment option for these diseases given their refractory status to conventional therapy. A large clinical trial is needed to. 11 It is plausible that blocking IL6 resulted in the improvement of this hyperinflammatory state especially in patients with baseline higher levels of IL6.
Tocilizumab is an IL-6 receptor monoclonal antibody that is approved for the use of CAR-T associated cytokine release syndrome. Tocilizumab is a recombinant humanized anti-IL-6 receptor monoclonal antibody that is approved by the FDA for use in patients with rheumatologic disorders and cytokine release syndrome CRS induced by chimeric antigen receptor T cell CAR T-cell therapy. 7 9 12 Ongoing randomized control trials will allow for further evaluation of this promising therapy.
The first approved medication in this class tocilizumab Actemra is an antibody directed against the IL6-receptor. Tocilizumab is a humanized monoclonal antihuman interleukin-6 IL-6 receptor antibody that may reverse IL-6-induced suppression of CYP3A4 activity. RoActemra or Actemra is a recombinant humanized monoclonal antibody that acts as an interleukin 6 IL-6 receptor antagonist.
In 12 patients with rheumatoid arthritis exposure to simvastatin was significantly reduced at 1 and 5 weeks after tocilizumab infusion 95. A Novel Humanized Anti-Interleukin 6 Receptor Antibody for the Treatment of Patients With Rheumatoid Arthritis. The first interleukin-6-receptor inhibitor.
In fact in COVID19 patients treated with tocilizumab IL6 levels are significantly elevated which are supportive of the cytokine storm. The second siltuximab Sylvant is directed against IL-6 itself. Below we share summaries of a series of studies evaluating the ability of tocilizumab to prevent clinical worsening and death of COVID-19 patients.
Tocilizumab a novel IL-6R inhibitor may be beneficial for the treatment of RA in patients who do not respond to methotrexate or disease-modifying antirheumatic drugs. Tocilizumab is a humanized monoclonal antihuman interleukin-6 IL-6 receptor antibody that may reverse IL-6-induced suppression of CYP3A4 activity. Given that tocilizumab functions by acting as a competitive inhibitor of the IL-6 receptor IL-6R increased serum IL-6 levels after its administration are likely caused by inhibition of IL-6Rmediated clearance as well as the endogenous production of IL-6 with ongoing disease activity.
The median time to improvement was 60 days 95 CI 50 to 60 in the tocilizumab group and 50 days 95 CI 40 to 70 in the placebo group. Siltuximab is approved for treatment of human immunodeficiency virus-negative and HHV-8 -negative patients with multicentric Castlemans disease. Tocilizumab binds to the interleukin-6 receptor IL-6R and thereby blocks signaling of the pro-inflammatory cytokine IL-6.
A study published in this issue of the Journal analyses the use of the IL-6 receptor blocker tocilizumab in 112 patients with RA in Germany and suggests a higher level of infections than those seen in clinical trials 2. Initial studies and all authority assessment reports state that tocilizumab is effective in humans but cannot bind to the murine or rat IL-6R and thus not block IL-6 signaling in the mouse. In 12 patients with rheumatoid arthritis exposure to simvastatin was significantly reduced at 1 and 5 weeks after tocilizumab infusion 95.
As a brief recap the IL-6 antagonist tocilizumab TCZ has now been studied as a therapeutic intervention for COVID-19 in at least 7 trials to our knowledge. Tocilizumab a monoclonal antibody that blocks IL-6 and is used to treat rheumatoid arthritis has been examined as a potential treatment for COVID-19. Clinical studies with tocilizumab a humanized monoclonal anti-IL-6 receptor antibody have been undertaken to explore this option.
