Myasthenia gravis is a chronic neuromuscular disorder. Soliris is indicated for the treatment of generalized myasthenia gravis gMG in adult patients who are anti-acetylcholine receptor AchR antibody positive.
 Fda Approves Soliris Eculizumab For The Treatment Of Patients With Generalized Myasthenia Gravis Gmg Business Wire
Fda Approves Soliris Eculizumab For The Treatment Of Patients With Generalized Myasthenia Gravis Gmg Business Wire
2 DOSAGE AND ADMINISTRATION.
Soliris myasthenia gravis. Soliris targets the underlying cause of gMG by preventing the immune system from becoming activated in an uncontrolled manner. On October 23 2017 the Food and Drug Administration FDA approved Soliris for generalized myasthenia gravis patients who are anti-acetylcholine receptor AchR antibody positive. Expanded use of the US.
It is not known if SOLIRIS is safe and effective in children with gMG. Food and Drug Administration-approved therapy Soliris eculizumab to treat myasthenia gravis MG could lead to a significant wholesale cost increase in 2018 and beyond. SOLIRIS is a prescription medicine used to treat adults with a disease called generalized myasthenia gravis gMG who are anti-acetylcholine receptor AChR antibody positive.
Further research into the. According to a new study the expanded use of Soliris for myasthenia gravis could lead to a significant increase in the wholesale cost for pharmacies and other distributors. People with hard-to-treat generalized myasthenia gravis gMG who respond to Soliris can reduce their use of immunosuppressive therapies a study shows.
Soliris is used to treat adults with myasthenia gravis a disease where the immune system attacks and damages muscle cells causing muscle weakness in whom other medicines do not work and who have a specific antibody in their body called AChR antibody. Myasthenia gravis community who have not seen a therapy approved for generalized myasthenia gravis in more than 60 years said Nancy Law Chief Executive Officer of the Myasthenia Gravis Foundation of America MGFA. The use of a worst-rank analytical approach proved to be an important limitation of this study since the secondary and sensitivity analyses results were inconsistent with the primary endpoint result.
People with refractory generalized myasthenia gravis gMG who responded to Soliris eculizumab were able to reduce the use of immunosuppressive therapies data from an extension study of the. SOLIRIS is a prescription medicine used to treat adults with a disease called generalized myasthenia gravis gMG who are anti-acetylcholine receptor AChR antibody positive. SOLIRIS is a prescription medicine used to treat adults with a disease called generalized myasthenia gravis gMG who are anti-acetylcholine receptor AChR antibody positive.
Soliris eculizumab Home HCP Site Soliris eculizumab treats anti-AChR antibody-positive gMG. Soliris is indicated for the treatment of adult patients with generalized Myasthenia Gravis gMG who are anti-acetylcholine receptor AchR antibody positive. It is particularly significant that this approval of Soliris will provide.
The humanized monoclonal antibody eculizumab Soliris is a complement inhibitor indicated for use in anti-acetylcholine receptor AChR antibody-positive adults with generalized myasthenia gravis gMG in the USA refractory gMG in the EU or gMG with symptoms that are difficult to control with high-dose IVIg therapy or PLEX in Japan. AchR antibodies inhibit acetylcholine a neurotransmitter that sends messages between nerve cells leading to loss of muscle function. The change in the MG-ADL score was not statistically significant between eculizumab and placebo as measured by the worst-rank analysis.
See Important Safety Information including Boxed Warning and full Prescribing Information. This approval represents the first FDA approval for this condition in over 60 years and provides an innovative treatment for a subset of myasthenia gravis. Alexion Pharmaceuticals announced Oct.
23 2017 that the US. Food and Drug Administration FDA has approved eculizumab brand name Soliris as a treatment for adult patients with generalized Myasthenia Gravis gMG who are anti-acetylcholine receptor antibody-positive. It is not known if SOLIRIS is safe and effective in children with gMG.
It is not known if SOLIRIS is safe and effective in children with gMG. SOLIRIS is the first and only complement inhibitor approved for adults with anti-acetylcholine receptor antibody-positive AChR generalized Myasthenia Gravis gMG a chronic and debilitating neuromuscular disorder. Eculizumab was well tolerated.
Soliris eculizumab may be the most effective and well-tolerated immunotherapy for patients with myasthenia gravis MG according to an analysis of multiple studies. FDA Approves Soliris to Treat Generalized Myasthenia Gravis Summary. SOLIRIS is the first and only complement inhibitor approved for the treatment of adult patients with gMG who are AChR.
This is a landmark day for the members of the US.
 Adding Multimedia Alexion Receives Fda Approval Of Soliris Eculizumab For The Treatment Of Adults With Neuromyelitis Optica Spectrum Disorder Nmosd Who Are Anti Aquaporin 4 Aqp4 Antibody Positive Business Wire
Adding Multimedia Alexion Receives Fda Approval Of Soliris Eculizumab For The Treatment Of Adults With Neuromyelitis Optica Spectrum Disorder Nmosd Who Are Anti Aquaporin 4 Aqp4 Antibody Positive Business Wire
Fda Soliris Is Approved To Treat Generalized Mg In The United States Muscular Dystrophy Association
 Soliris Eculizumab Hcp Mechanism Of Action
Soliris Eculizumab Hcp Mechanism Of Action
 Myasthenia Gravis Approval Will Boost Soliris Revenues
Myasthenia Gravis Approval Will Boost Soliris Revenues
 Fda Will Review Soliris For Myasthenia Gravis Muscular Dystrophy Association
Fda Will Review Soliris For Myasthenia Gravis Muscular Dystrophy Association
 Long Term Safety And Efficacy Of Eculizumab In Generalized Myasthenia Gravis Muppidi 2019 Muscle Amp Nerve Wiley Online Library
Long Term Safety And Efficacy Of Eculizumab In Generalized Myasthenia Gravis Muppidi 2019 Muscle Amp Nerve Wiley Online Library
 Argenx Antibody Proves Effective To Treat Myasthenia Gravis
Argenx Antibody Proves Effective To Treat Myasthenia Gravis
 Pdf Eculizumab A Review In Generalized Myasthenia Gravis Semantic Scholar
Pdf Eculizumab A Review In Generalized Myasthenia Gravis Semantic Scholar

 Proposed Algorithm For Treatment Of Myasthenia Gravis 15 Presence Of Download Scientific Diagram
Proposed Algorithm For Treatment Of Myasthenia Gravis 15 Presence Of Download Scientific Diagram
 James Howard M D The Role Of Complement In Myasthenia Gravis Youtube
James Howard M D The Role Of Complement In Myasthenia Gravis Youtube
 Alexion Crowns Myasthenia Gravis As Its Best Soliris Launch Ever Fiercepharma
Alexion Crowns Myasthenia Gravis As Its Best Soliris Launch Ever Fiercepharma
 Safety And Efficacy Of Eculizumab In Anti Acetylcholine Receptor Antibody Positive Refractory Generalised Myasthenia Gravis Regain A Phase 3 Randomised Double Blind Placebo Controlled Multicentre Study The Lancet Neurology
Safety And Efficacy Of Eculizumab In Anti Acetylcholine Receptor Antibody Positive Refractory Generalised Myasthenia Gravis Regain A Phase 3 Randomised Double Blind Placebo Controlled Multicentre Study The Lancet Neurology
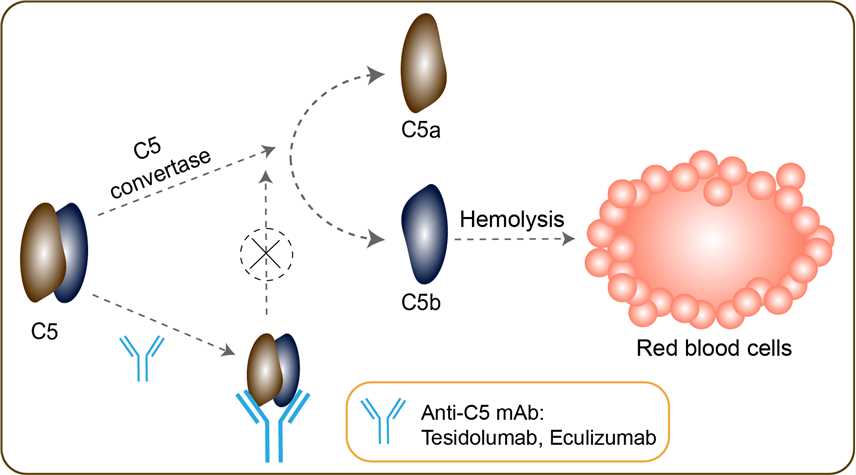 Eculizumab Overview Creative Biolabs
Eculizumab Overview Creative Biolabs

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.