Bleach contains chlorine in a state of oxidation I it is therefore a strong and economic oxidant. This bleaching power has the composition of calcium hypochlorite calcium hydroxide and calcium chloride.
 2 Composition Of Bleaching Products Download Table
2 Composition Of Bleaching Products Download Table
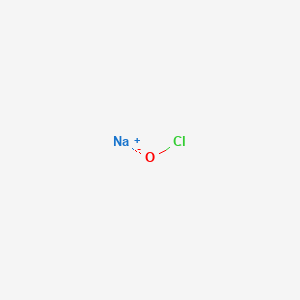
It consists of hypochlorite anion and sodium cation.
Chemical composition of bleach. Bleaching powder is the next popular type of chlorine based bleach. Wood and animal fibres are bleached by acidic reducing agents such as sulfur dioxide. These bleaches are made by bubbling chlorine gas through a solution of sodium hydroxide lye or calcium hydroxide quicklime.
It is also known as liquid bleach. It is prepared by electrolysis with minimal separation between the anode and cathode. Compared to sodium hypochlorite this powder bleach has more chlorine in it and tends to be more stable.
To prevent this from happening commercial bleaches leave. Sodium hypochlorite in 05 wv solution is called Dakins solution and is used as an antiseptic to clean infected topical wounds. They have the formula NaClO.
Its chemical formula is NaClO composed of one sodium Na atom one chlorine Cl atom and one oxygen O atom. The chemical formula of the active ingredient hypochlorite is ClO. The chemical formula of household bleach is NaClO.
Other ingredients include ammonium persulfate potassium persulfate and sodium persulfate. Chlorine gas can be released if the bleach is mixed with an acid. Chlorine bleach is made by mixing chlorine and caustic soda sodium hydroxide in a kind of reversal of the chlor-alkali process.
The chemical formula of chlorine bleach or sodium hypochlorite is NaClO. The chemical composition of bleach is Sodium hypochlorite. Household bleach is actually a mixture of chemicals Its main constituent is a solution of 3-6 sodium hypochlorite NaOCl which is mixed with small amounts of sodium hydroxide hydrogen peroxide and calcium hypochlorite.
Most hair bleach contains between 6 percent and 10 percent hydrogen peroxide. Sodium Hypochlorite is a chlorine compound often used as a disinfectant or a bleaching agent. Though household bleach generally all has the same formula in chemistry bleaching can be done with a number of different substances including hydrogen peroxide.
Sodium hypochlorite NaOClis formed when chlorine is passed into cold and dilute sodium hydroxide solution. It is commonly referred to as bleach because it is the active ingredient in bleach. Its chemical name is sodium hypochlorite.
It usually appears as a pale greenish yellow dilute solution. Chemical Reaction Ordinary household bleach contains sodium hypochlorite which reacts with ethanol or isopropyl alcohol to produce chloroform CHCl 3 hydrochloric acid HCl and other compounds such as chloroacetone or dichloroacetate. Sodium hypochlorite is a chemical compound with the chemical formula NaClO.
Hydrogen peroxide is the bleaching agent that reacts with hair to remove color. Persulfates are added to hair bleach to increase the rate of the chemical reaction. However they are both used in many applications in our lives.
Chemical composition of bleach Sodium hypochlorite or sodium hypochlorite are the chemical compounds know as bleach. In the pulp and paper industry chlorine dioxide hydrogen peroxide sodium peroxide sulfur dioxide sodium bisulfite and sodium hydrosulfite are commonly used.
 Sodium Hypochlorite Bleach Swimming Pools Cleaning Products Compound Interest
Sodium Hypochlorite Bleach Swimming Pools Cleaning Products Compound Interest
 Sodium Hypochlorite Structure Uses Formula Biology Class 2021 Video Study Com
Sodium Hypochlorite Structure Uses Formula Biology Class 2021 Video Study Com
:max_bytes(150000):strip_icc()/aceticacid-56a129995f9b58b7d0bca2c4.jpg) Chemical Composition Of Vinegar
Chemical Composition Of Vinegar
 Chemical Makeup Of Bleach Saubhaya Makeup
Chemical Makeup Of Bleach Saubhaya Makeup
:max_bytes(150000):strip_icc()/Potassium-chlorate-composition-58a3b5a43df78c475882bbb6.png) Make Potassium Chlorate From Bleach And Salt Substitute
Make Potassium Chlorate From Bleach And Salt Substitute
 What Is The Actual Formula Of Bleaching Powder Quora
What Is The Actual Formula Of Bleaching Powder Quora
 Compound Chemical Structure Of Chlorine Bleach Page 6 Line 17qq Com
Compound Chemical Structure Of Chlorine Bleach Page 6 Line 17qq Com
 Chemical Makeup Of Bleach Saubhaya Makeup
Chemical Makeup Of Bleach Saubhaya Makeup
 Making Bleach As Chemistry Youtube
Making Bleach As Chemistry Youtube
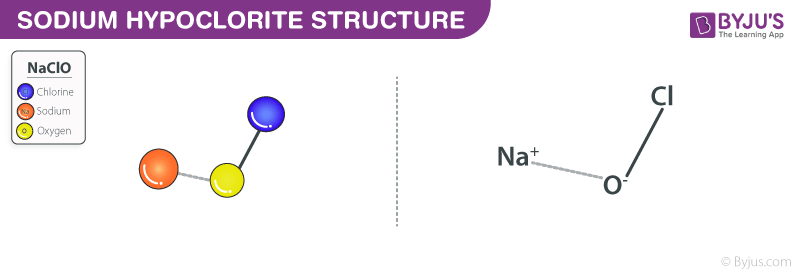 Sodium Hypochlorite Naclo Structure Molecular Mass Properties Uses
Sodium Hypochlorite Naclo Structure Molecular Mass Properties Uses
How Bleach Is Made Material Manufacture Making History Used Components Steps Product Industry



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.