Originally Boostrix was licensed in 2005 for persons aged 10 through 18 years but in 2008 FDA approved an expanded age indication for Boostrix to include persons aged 19 through 64 years. Download Boostrix IPV CMI PDF Product Information.
 Boostrix Vaccine For Whooping Cough Tetanus And Diphtheria Clinical Trials Arena
Boostrix Vaccine For Whooping Cough Tetanus And Diphtheria Clinical Trials Arena
Download Boostrix IPV PI PDF.

Boostrix age range. 20 IU tetanus toxoid. Revised December 2016 Author. Vaccination of children 1between 4 to6 years of age with BOOSTRIX in study APV-118 ATP.
7 rows When feasible Boostrix GSK should be used for adults 65 years and older. They include one dose only in adolescence or adulthood eg. The primary immunisation schedule is one 05mL dose given at 2 4 and 6 months of age.
Who did not complete DTaP series and adults 65 years and older HepA Pediatric Vaqta Havrix 1 year 18 years Adult Vaqta Havrix 19 years No upper limit. Tdap Boostrix 10 years No upper limit Adacel 10 years 64 years ACIP recommends Tdap for children 7-10 yrs. What Tdap vaccine should pregnant women receive.
Vaccinees generally reported a low incidence of severegrade 3 solicited local and general adverse events during the 1-month postvaccination periodCurrent recommendations for dTpa usage vary from country to country. Boostrix is a three-in-one vaccine for protection against whooping cough tetanus and diphtheria. Currently Boostrix is the only Tdap vaccine indicated in patients 65 years of age and older.
3 State -supplied 9vHPVvaccine is recommended for routineuse in males and females beginning at 1112 years with catch up vaccination through 18 years of age. 8 µg pertussis toxoid. Registered as a booster in people aged 4 years.
Boostrix has the broadest age range for a Tdap vaccine of ten years and older. Australia Canada France Switzerland and the US one dose at preschool age and one in adolescence Germany and. Children are typically given a dose of Boostrix at age 11 or 12 years.
Meningitec MenCCV Menjugate Syringe MenCCV and Menitorix Hib-MenCCV are registered for use from 6 weeks of age. If your child misses a dose If your child misses a. The mean number of years since the last ers in current vaccination programmes.
Boostrix diphtheria tetanus acellular and pertussis adult vaccine - also called Tdap is used to help prevent tetanus diphtheria and pertussis in people who are at least 10 years old. Boostrix is FDA-approved for use in children ages 10 years and older. FDA has now expanded the age indication to include persons aged 65 years and older.
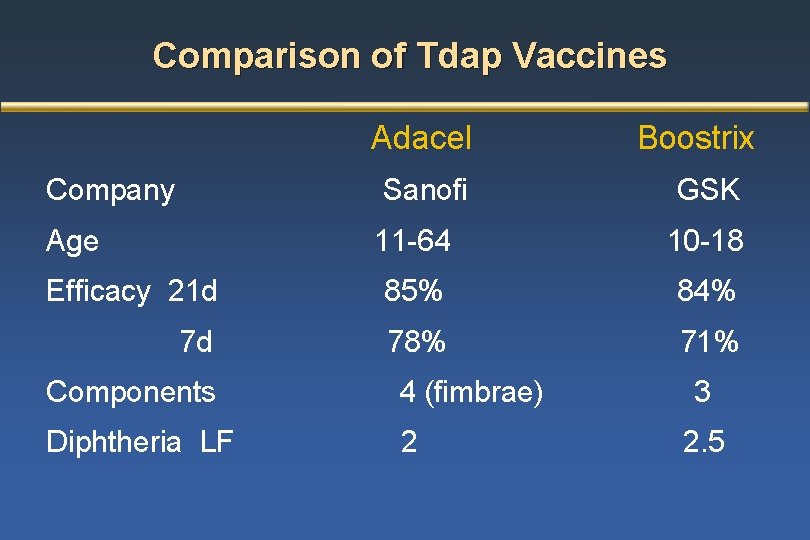
While Adacel is indicated for patients 10-64 years old Boostrix is indicated for patients 10 years of age and older. The recommended age for use of 4vMenCVs varies between vaccine brands. DTpa diphtheria-tetanus-acellular pertussis combination vaccine reduced antigen formulation Each 05 mL monodose vial or pre-filled syringe contains.
2 IU diphtheria toxoid. The number and range of vaccines and doses that are eligible for NIP funded catch-up is different for people aged less than 10 years and those aged 1019 years. NeisVac-C MenCCV is registered for use from 8 weeks of age.
Most people in this age group require only one Tdap shot for protection against these diseases. The Product Information is intended to assist healthcare professionals make decisions about treatment options and provide advice on the appropriate use of a medicine to patients. A booster dose may be given at 15 months to 6 years of age.
All people aged less than 20 years are eligible for free catch-up vaccines. The biggest difference is the age that the vaccines are recommended for. BOOSTRIX is indicated for active booster immunization against tetanus diphtheria and pertussis in individuals aged 10 years and older.
This information only applies to GSK products available in Australia. For active booster immunization against tetanus diphtheria and pertussis in individuals 10 years of age and older. Boostrix was approved for people in the age group of 65 years and older in 2011.
Boostrix and Boostrix IPV could be used in routine participating subjects 40 years of age was 203 years standard adult vaccination programmes or could replace existing Td boost- deviation 132 years. Product Information Package Insert - Boostrix. Read more about NIP NIP catch-up immunisations.
The safety profile presented in Table 1 is based on data from clinical trials where Boostrix-IPV was administered to 908 children from 4 to 8 years of age and 955 adults adolescents and children from 10 to 93 years of age.
Https Www Ema Europa Eu Documents Variation Report Infanrix Hexa H C 296 P46 131 Epar Assessment Report En Pdf
 Tetanus Diphtheria Pertussis Vaccines Download Table
Tetanus Diphtheria Pertussis Vaccines Download Table
Https Www Fda Gov Media 76633 Download
 Table 5 From Adolescent Immunizations Semantic Scholar
Table 5 From Adolescent Immunizations Semantic Scholar
 Boostrix Vaccine For Whooping Cough Tetanus And Diphtheria Clinical Trials Arena
Boostrix Vaccine For Whooping Cough Tetanus And Diphtheria Clinical Trials Arena
Https Www Ema Europa Eu Documents Variation Report Infanrix Hexa H C 296 P46 131 Epar Assessment Report En Pdf
 Pertussis Awareness And Prevention W Michael Brown Md
Pertussis Awareness And Prevention W Michael Brown Md
 Boostrix Vaccine Guide National Vaccine Support Group
Boostrix Vaccine Guide National Vaccine Support Group
 Comparison Of Boostrix And Adacel Adsorbed Products Licensed In The Download Table
Comparison Of Boostrix And Adacel Adsorbed Products Licensed In The Download Table
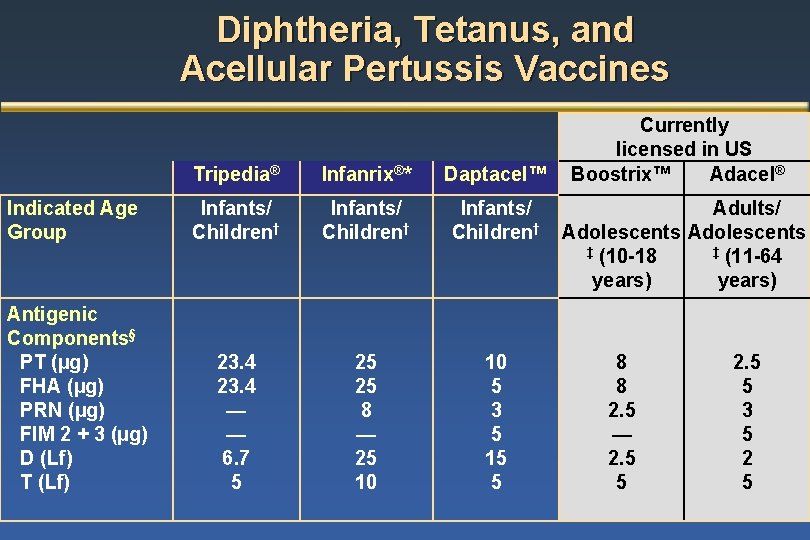
 Composition Of Dtap Vaccine And Tdap Vaccines By Manufacturer Download Table
Composition Of Dtap Vaccine And Tdap Vaccines By Manufacturer Download Table
 Boostrix 0 5 Ml Needleless Pre Filled Syringe Vaccine 10 Pack
Boostrix 0 5 Ml Needleless Pre Filled Syringe Vaccine 10 Pack
 Jkms Journal Of Korean Medical Science
Jkms Journal Of Korean Medical Science
 Pertussis Awareness And Prevention W Michael Brown Md
Pertussis Awareness And Prevention W Michael Brown Md


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.